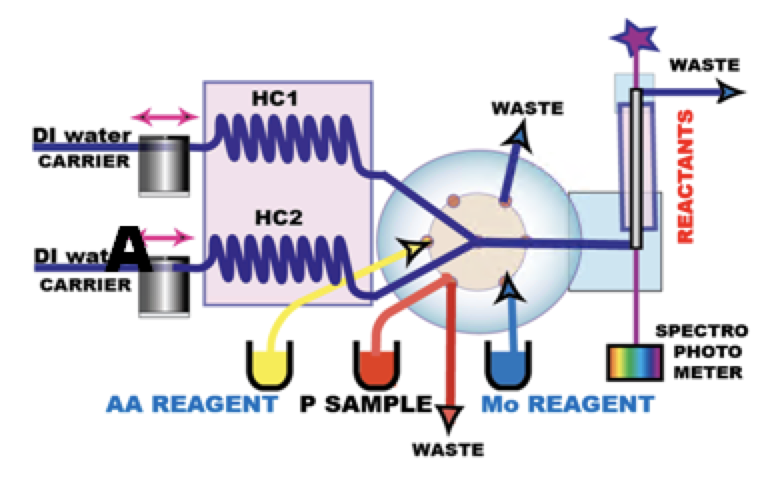
Instrumentation and Reagents
INSTRUMENTATION. Thermostated holding coils and flow cell of the miniSIA-2 instrument (A) were held at 22 C and the 20 cm long light path flow cell was used for all experiments discussed in this work.
FLOW PROGRAMMING, described in the next page (1.5.9.) is designed to mix AA reagent with Mo reagent in ratio 1+1 and the resulting mix to blend with P sample solution also in 1+1 ratio. Therefore the concentration of reactants in the flow cell is 1/4 of reagent input concentrations. Because it is the composition of reactants in the flow cell, that determines rates of reactions and their outcome, all concentrations denoted in molarities and normalities in [H+] diagrams and other figures, presented in the following sections, represent concentrations of reactants in the flow cell during incubation period.
REAGENTS were prepared in deionized water (DI), which was also used as carrier solution.
Ascorbic acid input reagent was prepared daily, by dissolving 1.5g of ascorbic acid followed by 1.5g of sodium dodecyl sulfonate in 50ml of DI water, resulting in 0.17M ascorbic acid, containing 3% of SDS, and thus 43mM AA and 0.75% SDS in the reactant mixture.
Molybdate input reagent was composed of various concentrations of ammonium molybdate (Mo), various concentrations of strong acids and the constant concentration of potassium antimony tartarate that resulted on 0.03mM KSb tartarate in the mixture of reactants in the flow cell.
Stock solution of ammonium molybdate (0.020M) was prepared by dissolving 2.5g of ammonium molybdate tetrahydrate in 100 mL DI water.
Stock solutions of sulfuric, hydrochloric and nitric acids were prepared by dilution of analytical grade concentrated acids. Their concentration was adjusted to 4.00 N and confirmed by titration with standard solution of sodium hydroxide.
Input concentrations of molybdate reagent were selected to yield 0.50, 1.0 and 1.5mM concentrations of molybdate in the reactant mixture, while normalities of strong acids were varied stepwise between 0.05N and 0.7N.
Input concentration of orthophosphate standard solutions were prepared by serial dilution of 1000 ppM P commercially available standard by DI. They are, in this work, referred to in ppB units in order to distinguish them from P concentrations in reactant mixture. Thus e.g. 31 ppB P yields 0.5microM P in the reactant solution, because P standards are diluted by reagents 1+1.